
Hsp70s and the small Hsps, on the other hand, adopt modular “clamps” for protecting extended hydrophobic structures in their targets. The Hsp60s adopt a barrel-like Anfinsen cage structure for sequestered folding of target proteins. Aside from their differences in size, the structures of these different classes are quite divergent. Further experimentation revealed several types of functions for different chaperone proteins, which may be attributed to the diversity of their structures.Ĭurrent structural information divides the chaperones into five major classes based on their observed molecular weights: Hsp60, Hsp70, Hsp90, Hsp104, and the small Hsps. The functions of these proteins were validated with the generation of recombinant versions of the proteins that performed their expected functions outside the original cellular environment. Expression of these proteins was increased with heat shock treatment, leading to their label as heat shock proteins (Hsps). These genes are bacterial homologs of Hsp60, Hsp10, Hsp70, Hsp40, and the nucleotide exchange factor for the Hsp70/Hsp40 machine. The disruption of groEL, groES, dnaK, dnaJ, and grpE was found to have deleterious effects for the growth of the bacteriophage. The first of these chaperone proteins was found by Sternberg in 1973 in studies of mutations that disrupted bacteriophage λ head formation. Ĭonsidering the dense population of the cytosol (average protein conc: 150 mg/mL), Finka and Goloubinoff proposed an inherent need to protect nascent polypeptides from “unwanted associations” that prevent the attainment of the functional protein fold. While this simple and elegant principle governs most biological systems, literature from both the distant and recent past have cited complications in the cellular environment that may disrupt the flow of genetic information. The central dogma of molecular biology states that genes are transcribed into messenger RNAs, which are then translated into the proteins that carry out cellular functions.
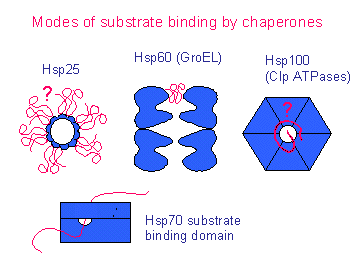
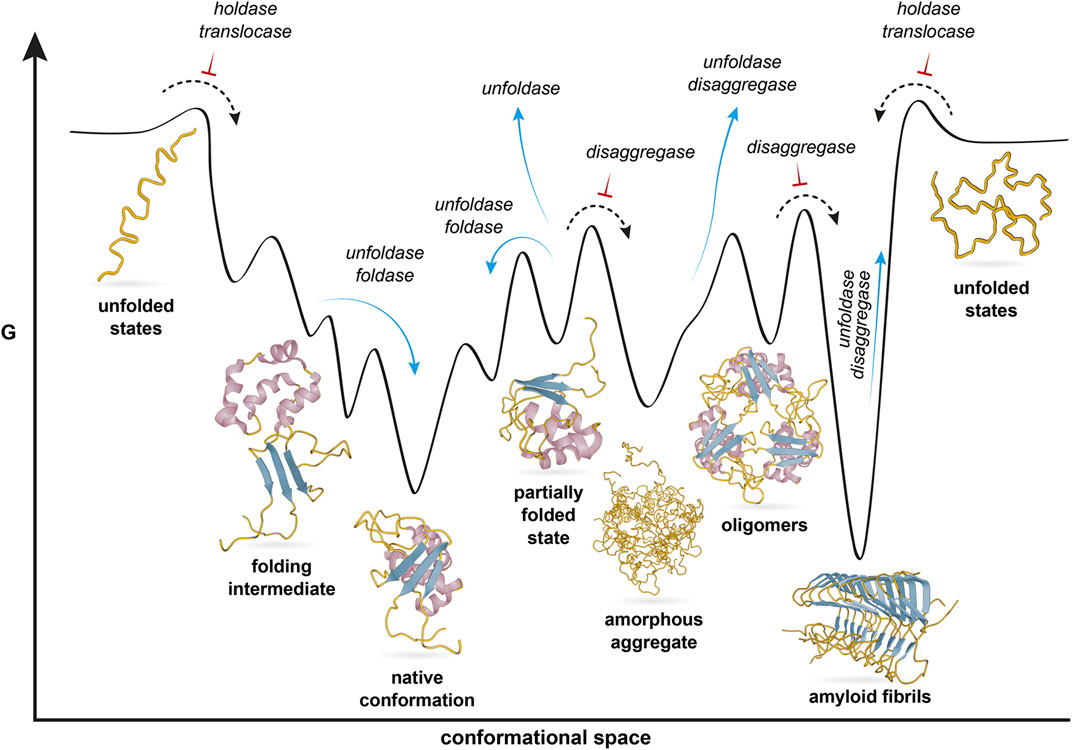
The structures of domains and the associated functions are discussed in order to illustrate the rationale for the proposed unfoldase function. When possible, it discusses the complete structures for these proteins, and the types of molecular machines to which they have been assigned.
It reviews the currently available molecular structures in the Protein Data Bank for several classes of Hsps (Hsp60, Hsp70, Hsp90, and Hsp104). This current article focuses on the resolved structural bases for these functions. Onto this is added specializations that allow the different family members to perform various cellular functions. The term “unfoldases” has been proposed, as this basic function is shared by most members of this protein family. However, neither label encompasses the breadth of these proteins’ functional capabilities. These chaperone proteins also increased in expression as a response to heat shock, hence their label as heat shock proteins (Hsps). These proteins’ ability to prevent unwanted associations led to their being called chaperones. Send us feedback about these examples.Thirty years ago a class of proteins was found to prevent the aggregation of Rubisco. These examples are programmatically compiled from various online sources to illustrate current usage of the word 'histone.' Any opinions expressed in the examples do not represent those of Merriam-Webster or its editors.
2020 In the nucleus, DNA is bound to proteins called histones to make a complex called chromatin, which in turn forms chromosomes. 2022 By binding to the H3 histone, serotonin and dopamine can regulate transcription of DNA into RNA and, as a consequence, the synthesis of specific proteins from them. 2023 Additionally, a variety of epigenetic changes (mediated, for example, by DNA methylation, histone modification and transfer of non-coding RNAs) also appear to increase with advancing maternal age (ref & ref). 2022 It is controlled by methylation changes to CpG islands on promoter regions, the remodeling of chromatin using histone modifications, and the alteration of gene expression through non-coding RNA expression. 2015 The histone in question is one known as PRDM2. Paul Haggarty, Discover Magazine, 13 Oct. Recent Examples on the Web In their new study, scientists at McGill University in Canada, used genetic engineering to alter the activity of one of the histone proteins that controls epigenetic processes – KDM1A histone lysine 4 demethylase – during the production of sperm in mice.


 0 kommentar(er)
0 kommentar(er)
